Health Minister Patty Hajdu who previously questioned the accuracy of antigen tests said the new Abbott device will take pressure off overrun testing sites and offer specific advantages for. On its website and in news releases Abbott maintains its test performs best in patients tested earlier post symptom onset.
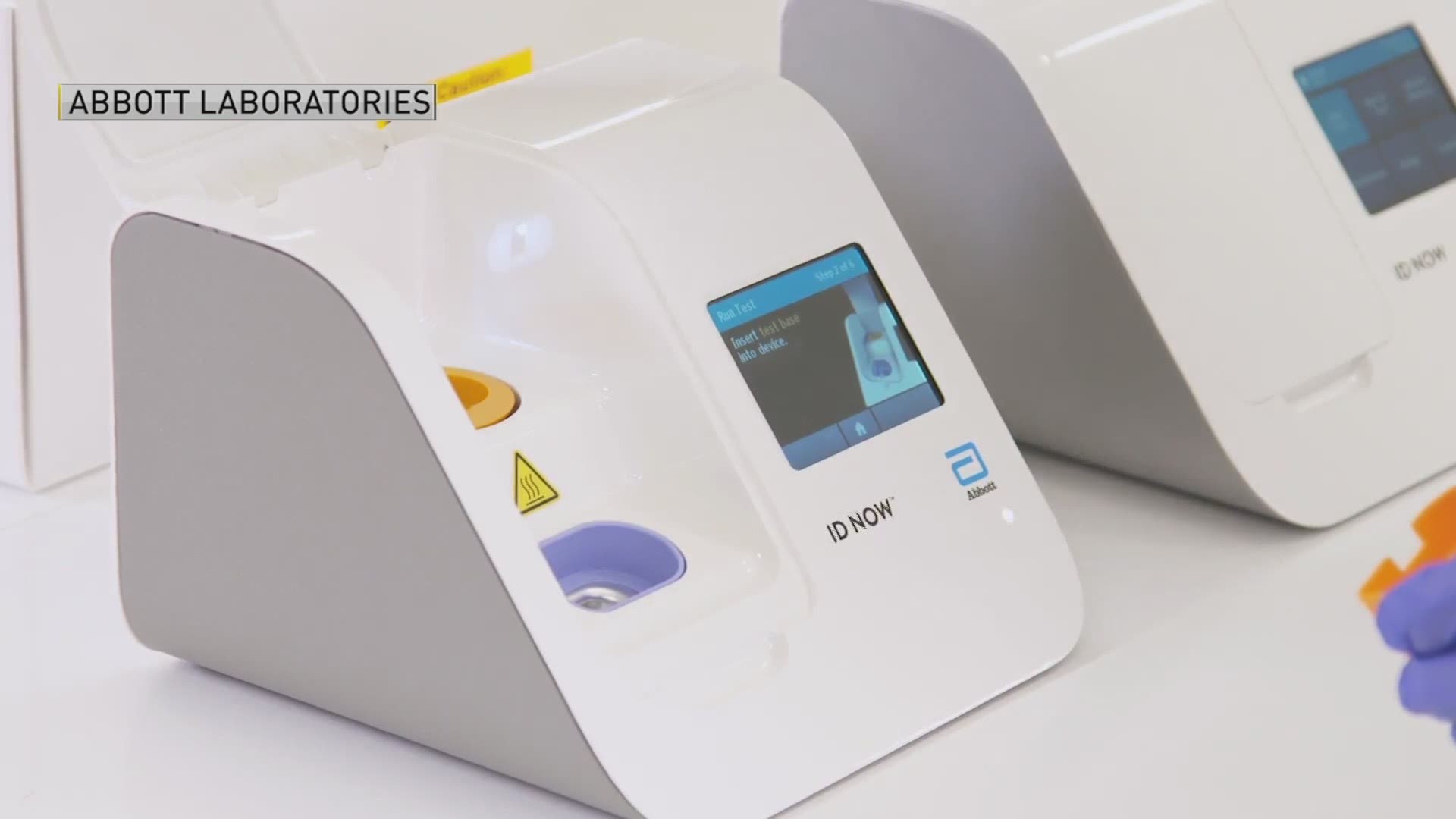 Fda Fast Tracks Covid 19 Testing Device Made In Maine Newscentermaine Com
Fda Fast Tracks Covid 19 Testing Device Made In Maine Newscentermaine Com
Shortly after Abbott completed its delivery of 150 million rapid antigen tests to the federal government for widespread distribution against the COVID-19.

Abbott rapid test accuracy. The BinaxNOW COVID-19 Ag Card 2 Home Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in direct anterior nasal nares swabs from individuals with or without symptoms or other epidemiological reasons to suspect COVID-19 infection when tested twice over three days with at least 36 hours between tests. Rapid antigen tests such as the Abbott BinaxNOW COVID-19 Ag Card BinaxNOW offer results more rapidly approximately 1530 minutes and at a lower cost than do highly sensitive nucleic acid amplification tests NAATs 1. Several independent research teams have called into question the accuracy of Abbotts ID NOW COVID-19 test a rapid point-of-care molecular diagnostic test designed to produce results in five minutes.
The Abbott rapid test can still be used to identify positive COVID-19 cases according to Stenzel though negative results may need to go through a secondary process to. Annons Abbott Panbio Rapid Antigenic Test. Abbotts rapid testing quickly identifies infectious patients for containment and its low-cost mass-production capabilities ensure those who need tests get them.
Annons Abbott Panbio Rapid Antigenic Test. Annons Agilent delivers better scientific and business outcomes for labs. We included test accuracy studies of any design that evaluated commercially produced rapid antigen or molecular tests suitable for a point-of-care setting minimal equipment sample preparation and biosafety requirements with results within two hours of sample collection.
Furthermore BinaxNOW can be used. Rapid antigen tests have received Food and Drug Administration FDA Emergency Use Authorization EUA for use in symptomatic persons 2 but data are lacking. The BinaxNOW COVID-19 Ag Card 2 Home Test is a lateral flow immunoassay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in direct anterior nasal nares swabs from individuals with or without symptoms or other epidemiological reasons to suspect COVID-19 infection when tested twice over three days with at least 36 hours between tests.
Previously antigen tests were thought to. Last week researchers at New York Universitys health system published a report showing accuracy problems with Abbotts rapid coronavirus test. FDA warns on accuracy of Abbott rapid COVID-19 test May 15 2020 By Nancy Crotti Abbotts ID Now test machine Image from Abbott After weeks of concerns about the.
Rapid tests such as Abbotts are generally less accurate than molecular diagnostic tests which are the industry gold standard but must be sent to a laboratory to produce results a process that. Agilent is a leader in the life sciences diagnostics and applied chemical markets. Food and Drug Administration issued an alert Thursday about the accuracy of Abbott Labs rapid Covid-19 test after researchers at New York.
FDA granted Abbott an emergency use authorization of the test in late March. Abbotts ID NOW COVID-19 test is promoted as delivering positive test results in five minutes and negative results in about 13 minutes. The Abbott rapid antigen test authorized in August reports a 971 percent sensitivity rate which is very promising.
:max_bytes(150000):strip_icc()/001_charter-speed-test-review-2626132-5c42293046e0fb00018a3c60.jpg)