The first generic version of the inhalation treatment Advair Diskus fluticasonesalmeterol powder was recently approved by the US. Advair fluticasone and salmeterol is a prescribed medicine used to stop air passage convulsions caused by such conditions as asthma and chronic obstructive.
 Wixela Generic Form Of Advair Now Approved
Wixela Generic Form Of Advair Now Approved
Plus in 2013 they gained FDA approval of a product once hailed as Super Advair now called Breo.
What is generic advair called. FDA approved the first generic version of blockbuster drug Advair fluticasonesalmeterol for asthma maintenance treatment in patients aged 4 and up and also for maintenance treatment and prevention of exacerbations in chronic obstructive pulmonary disease COPD. Advair fluticasone and salmeterol is a prescription medication used to treat airway spasms bronchospasms caused by asthma or chronic obstructive pulmonary disease COPD. The dosage is based on your medical condition.
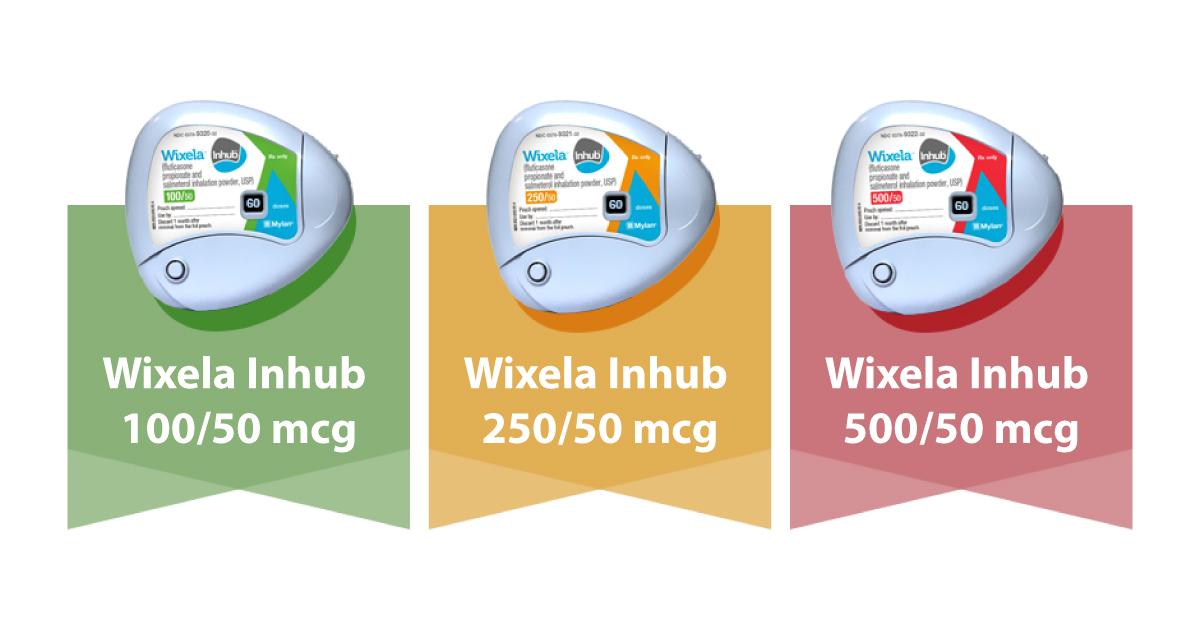
FDA authorizes Wixela Inhub. Advair contains a combination of fluticasone and salmeterol. Wixela Inhub is the first generic approved version of Advair Diskus.
Food and Drug Administration for chronic obstructive pulmonary disease COPD and asthma patients. Asthma and COPD patients who use the Advair Diskus got some good news last week when the FDA approved the first generic version of the respiratory medication. They are both dry powder inhalers that contain the same active and inactive ingredients.
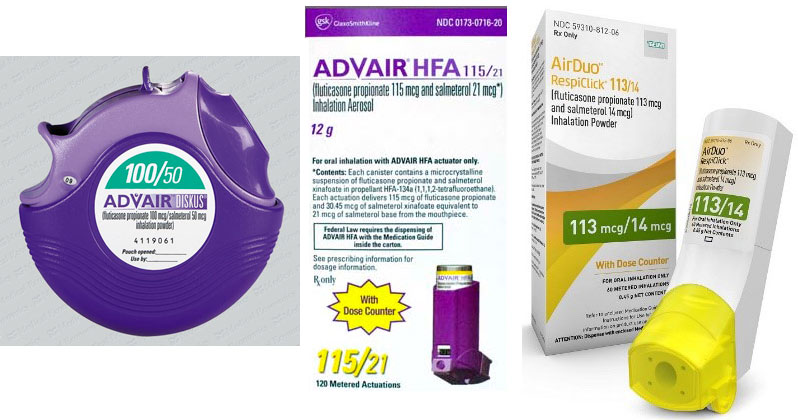
The Advair Diskus is available in 3 strengths of fluticasone propionate 100 250 and 500 microg and a fixed dose 50 microg of salmeterol. The lowest price for ordering advair diskus. It contains the same active drugs as Advair Diskus.
It comes in two forms -- the Advair Diskus and the Advair HFA inhaler. Mylan MYL secures an FDA approval for the first generic version of blockbuster COPD drug Advair Diskus. Advair Diskus Advair HFA AirDuo RespiClick.
Medically reviewed by Kaci Durbin MD. Compared to Advair which is one puff twice daily Breo is one puff once daily. So some asthmatics like this despite the high cost.
Mylan MYL secures an FDA. Advair Diskus is available in a generic form called Wixela Inhub. An authorized generic version of advair diskus is available without prescription.
Advair fluticasonesalmeterol is one of the most commonly prescribed inhalers for asthma and COPD. Canonsburg PA-based Mylans shares jumped 7 to 3082 at market close after yesterdays late-afternoon announcement and were trading at 3096 mid-morning today. Food and Drug Administration today approved the first generic of Advair Diskus fluticasone propionate and salmeterol inhalation powder for the twice-daily treatment of asthma in patients.
Asthma Policy Ethics Education. On GoodRx Advair is currently the most popular medication in a class of drugs known as beta-agonistcorticosteroid combination drugs. Last updated on Feb 2 2020.
For more details see the Advair vs. It is identical to the branded product and has exactly the same ingredients. Mylans Generic Version of Advair Diskus Wins FDA Approval.
Generic Advair Diskus is indicated for the twice-daily treatment of asthma in patients aged four years and older and maintenance treatment of airflow obstruction and reducing exacerbations in patients with COPD. Teva had previously introduced a fluticasonesalmeterol combination called AirDuo RespiClick but it did not gain the substitutable status that propelled Wixela. Fluticasone and salmeterol floo TIK a sone sal ME te rol Brand Name.
Then Mylans Wixela matched Advair closely enough that it was given approval as a substitutable generic meaning pharmacists can recommend that patients use it rather than the GSK product. Combination treatment with both ICS and LABA provides greater asthma control than increasing the ICS dose alone while at the same time reducing the frequency and perhaps the severity of exacerbations. This class also includes Breo Ellipta fluticasonevilanterol and AirDuo the other fluticasonesalmeterol.
1 Mylans product Wixela Inhub will be offered in 3 strengths at wholesale acquisition costs WACs. FDA has approved the first generic of GlaxoSmithKlines NYSEGSK blockbuster Advair respiratory drug this one by Mylan NSDQMYL six months after rejecting Mylans application. The first FDA-approved generic version of Advair Diskus GlaxoSmithKline GSK a fluticasone propionate and salmeterol inhalation powder product has been launched Mylan NV.
Fluticasone propionate is a corticosteroid medication that works by decreasing inflammation in the airways and Salmeterol is a long-acting beta-2-adrenergic agonist that works by reducing bronchoconstriction bronchodilator.


