We have reported regularly to the European Commission on how companies use non-animal approaches under the REACH Regulation The use of alternative to testing on animals for the REACH Regulation. Cruelty Free International does not agree with the narrow interpretation put.
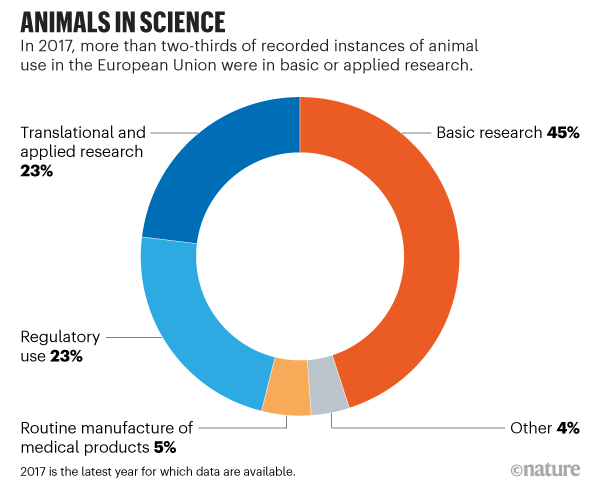 Animal Research Data Show Effects Of Eu S Tough Regulations
Animal Research Data Show Effects Of Eu S Tough Regulations
Depending on the product type its likely toxicity.

Animal testing regulations. It applies to all systems acquired under the auspices of the AR 70series. Animal testing also known as animal experimentation animal research and in vivo testing is the use of non-human animals in experiments that seek to control the variables that affect the behavior or biological system under studyThis approach can be contrasted with field studies in which animals are observed in their natural environments or habitats. Animal studies whether for the development or production of new medicines for physiological studies for studying environmental effects or for the testing of chemicals or new food additives has to be carried out in compliance with EU legislation.
Government regulations in many countries require toxicity testing on animals as a condition for the importation or sale of pesticides industrial chemicals drugs medical devices vaccines genetically modified foods and some consumer products. Following a 2020 European Chemicals Agency ECHA Board of Appeal ruling it was decided that even for sole use cosmetics ingredients animal testing may be required for EU REACH. Animal Testing Cosmetics Consumers and manufacturers sometimes ask about the use of animals for testing cosmetics.
Research on great apes orangutans gorillas chimpanzees and bonobos was banned in 1986 and animal. The following information addresses the legal requirement for cosmetic safety. Department of Agriculture USDA has set forth federal regulations governing the care and use of laboratory animals in biomedical research that are more extensive than those covering human subjects.
In the United States animal testing at least as it applies to vertebrates is largely regulated by the Animal Welfare Act of 1966 AWA. Animal Testing Has the Strict Regulations Various animal testing essay examples demonstrate that there are strict regulations for experimenting with animals nowadays. The UK has some of the strictest animal research regulations in the world guided by the Animals Scientific Procedures Act 1986 ASPA.
Government regulations often require numerous different animal-poisoning tests to assess the hazards of a single new chemical pesticide or medicinal product. This regulation implements the test and evaluation policies and proce-dures contained within DODD 320011 DODD 500001 DODI 500002 DODI 513417 and AR 70 1 and specifically prescribes implementing policies for the Armys testing and evaluation program. The Committee for the Purpose of Control and Supervision of Experiments on Animals CPCSEA Principle 1.
Animal tests are only needed if there is no alternative way to demonstrate safe use. This regula-tion implements test and evaluation policy. The law requires companies to use alternative methods whenever possible so companies should only ever test on animals as a last resort.
Fees Testing Laboratory IVD. The federal law called the Animal Welfare Act AWA sets high standards of care for lab animals. To obtain this information companies need to test chemicals either on animals or by using alternative methods.
These reports have confirmed that the most important tool to avoid unnecessary animal testing ie. A topical issue of present-day life is the protection of animal rights and numerous organizations control the situation. Data sharing is working well.
The AWA is designed to provide a minimum standard of care and treatment for animals while ensuring that the regulations do not. The AWA is enforced by the Animal and Plant Health Inspection Service APHIS of the United States Department of Agriculture USDA. It is limited to warm-blooded animals and it does not cover many of those most notably excluding mice rats and.
Purpose behind carrying out the experiments. Avoid animal experimentation if an alternative exists. It applies to all research involving animals in the US but it does not apply to all animals.
A comprehensive system of government oversight is in place to regulate the use of animals in testing and research. Clinical Trials Marketing Authorisation Human Marketing Authorisation Veterinary Approvals. Information about their properties.
The truth behind animal testing rights and regulations in India. The Animal Welfare Act signed into law in 1966 and updated by several amendments is the only federal law that regulates the treatment of animals in research exhibition and transport and by dealers. Batch Testing Human Batch Testing Veterinary Inspections Reference Material.
2011 2014 and 2017. The Public Health Service Policy on Humane Care and Use of Animals PHS Policy and USDA Animal Welfare Regulations AWRs provide the primary regulatory basis for the existence and function of the Institutional Animal Care and Use Committee IACUC which must be established at any institution that receives PHS funding for vertebrate animal-based research andor which conducts animal-based research involving vertebrate animal species regulated by the USDA AWRs. Appropriate sedation and anaesthesia.
